Published in our 2016-17 issue.
100 years after its discovery, a forgotten antibacterial treatment re-emerges
The advent of antibiotics in the early 20th century was arguably one of the most significant chemotherapeutical advances in human history, and ushered in a “golden age” of significantly improved health throughout the West. These drugs treated age-old diseases like pneumonia, dysentery and cholera, and dramatically improved the safety of surgical and medical procedures. But this golden age of antibiotic efficacy is quickly ending. The emergence and rapid spread of multidrug-resistant bacteria—most notably, methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococci, and carbapenem-resistant Enterobacteriaceae—suggests that a “post-antibiotic” era is approaching. In order to avert the onset of dramatically elevated health risks due to bacterial infection in this new era of antibiotic inefficacy, it is in the best interest of the medical community to seek out an alternative treatment option.
One of the most promising candidates to fill this niche is something that has been forgotten as a viable antibacterial therapy by Western medicine for nearly a century. Although initially outcompeted by penicillin, it has recently been revisited by many researchers in the U.S. and Europe for its potential to supplement antibiotics as they become less effective in the face of drug-resistant bacteria. This reemerging therapeutic strategy, which turns 100 years old in 2017, goes by the name bacteriophage therapy.
What are phages, and what is phage therapy?
Bacteriophages (or “phages”) are a diverse group of obligate bacterial viruses. Many phages, known as virulent or lytic phages, propagate by invading the host bacteria and hijacking its replication machinery in order to mass-produce phage progeny (see Figure 1). This ultimately bursts open the cell, potentially freeing 100 or more new virions.
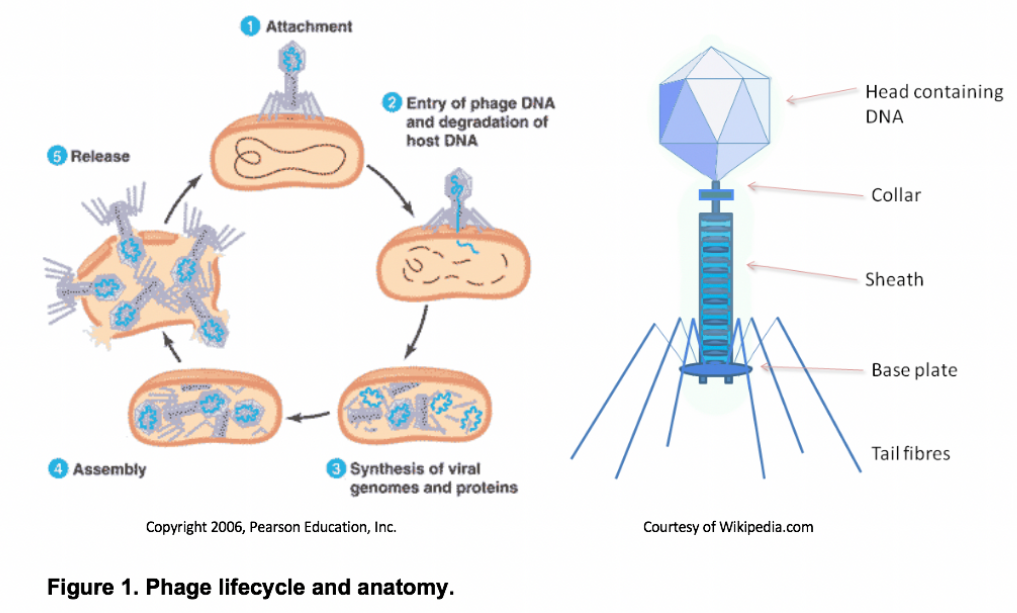
Although phages are structurally and genetically simple, they have proven to be remarkably successful throughout the biosphere. Recent evidence suggests that phages are the most abundant biological entities on the planet, with roughly 10 phage particles existing for every bacterium or 1031 particles in total1, 2. To put this in perspective, there are about 10 orders of magnitude more phages on Earth than there are stars in the universe2. These phages can be found wherever bacteria thrive, such as in the soil, groundwater, sewage, and even within larger organisms. This overwhelming phage abundance enables the rapid discovery of novel phages, including those naturally capable of infecting human pathogens. This inevitably leads to the idea of phage therapy, or using naturally-occurring phages as therapeutics capable of treating human infections. Proponents of phage therapy revel in the prospect of being able to isolate clinically-significant therapeutics from the environment around us.
In addition to the fact that novel phages are often easy to isolate due to their virtually infinite abundance and diversity, using phages in the clinic to treat human infections has a variety of intrinsic advantages. Several are discussed here.
1. Narrow host specificity. Phages are generally very specific with regard to the bacteria they target; that is, they are usually only capable of infecting a single species or even a single strain3, 4. In contrast to broad-spectrum antibiotics that target a wide range of species—often including favorable body flora—phages are capable of waging pinpoint attacks on pathogenic bacteria while leaving “good” bacteria unaffected. As a result, phage therapy often yields fewer side effects than antibiotic therapy5.
2. Safe for use in humans. Although highly effective in parasitizing bacterial cells, phages are not known to affect human cells. This can be explained by a variety of molecular barriers to infection, including structurally different outer-membrane proteins (which normally allow the phage to enter), incompatibility between viral mRNA and eukaryotic translation machinery, an inability to degrade host DNA, and incompatibility between phage lysis proteins and eukaryotic membranes. These factors combined strongly suggest that phages will remain adapted to target bacteria specifically, and not human cells. In addition, properly purified phage preparations are not known to induce anaphylaxis and are non-toxic7, 8.
3. Rapid development. As was discussed earlier, novel clinically-relevant phages can be isolated from the environment around us with relative ease. In contrast to antibiotics, which often require a decade or more of concerted development efforts and often accrue $1 billion or more in development costs, phages can—quite literally—be found in the soil beneath our feet. It is not out of the ordinary for novel phage-based therapeutics to be developed and mobilized in a year or less with very little cost.
4. Auto dosing. In contrast to antibiotic therapy, which slowly diffuses into human tissues and becomes ineffectual over time, phage-based therapeutics propagate at the site of infection by infecting and replicating within the infectious cells. The local therapeutic dose at the infection site thus increases through a phenomenon called “auto dosing”, which extends phages’ tissue penetration and therapeutic half-life5, 6.
5. Manageable phage resistance. Although phage resistance—like antibiotic resistance—is an inevitable result of using phages in the clinic, phage resistance is much more manageable. For one, because phages are naturally capable of coevolving with their hosts to counteract the accumulation of resistance, it is possible to “reactivate” ineffectual phages (i.e. those no longer effective in the face of host resistance) via in vitro evolution11, 12. This is not the case for antibiotics, which cannot easily be recovered after encountering resistance. Additionally, because phages often target receptors on host cells that are responsible for critical cellular functions, it is often difficult and/or costly for the cells to mutate or remove these receptors in the attempt to resist phage infection. Thus, resistant cells are often found to be sickly and less virulent than their non-resistant predecessors9, 10.
Despite these advantages, phage therapy has not been actively pursued by Western allopathic medicine for almost a century. In order to better understand why, we must take a look back into the rich history of the field of phage therapy.
The forgotten miracle cure
Proposed in 1917 by the French microbiologist Felix d’Herelle, phage therapy was on track to become the preeminent antibacterial therapy of the 21st century. Phages were relatively simple to isolate from the environment, and produced negligible side effects in those patients treated (unlike with other antibacterial drugs used at the time)13, 14. During the 1920s, d’Herelle and others boasted great successes in the treatment of age-old infections like Shigella dysenteriae, Vibrio cholerae, Yersinia pestis, Streptococcus spp., and Pseudomonas aeruginosa using phage therapy14. The technique spread across Europe, and by 1930 phage therapy had become a beacon of hope for the medical community.
However, the early success of phage therapy was quickly eclipsed by small-molecule antibiotics like penicillin15. Unlike phages, antibiotics were found to be extremely broad-spectrum, meaning that a single drug could be used to treat a variety of infections. This ultimately made antibiotics more attractive to physicians, because these drugs could be utilized effectively against seemingly every new infection that entered the clinic. On the other hand, because phages exhibited narrow host specificity, they often needed to be assembled into cocktails containing many different phages in order to achieve a level of breadth comparable to that of early antibiotics1, 16.
In addition to being more attractive to physicians, antibiotics were also more attractive to the pharmaceutical industry. Because a single antibiotic could treat a broad spectrum of infections, these drugs were much more scalable than phages and could be mass-produced. Additionally, companies wishing to market phage therapy ran into a number of regulatory obstacles, including difficulties in patenting individual phage preparations1, 23.
Despite these obstacles, certain labs and physicians—especially in Eastern Europe and the Soviet Bloc—continued their work with phage therapy and ultimately developed immense collections of phages capable of infecting many common human pathogens. As antibiotics decline in efficacy, this field is now re-emerging into mainstream clinical microbiology.
Phage therapy in the 21st century
Recent technological advances have opened new frontiers for phage therapy. Phages have been applied recently in various forms: as whole lytic phages, as genetically-modified phages, and as sources for endolysins and other antibacterial enzymes. These new applications illustrate the potential for a reemergence of phage therapy altogether in the coming decades.
Decreasing antibiotic efficacy over the past several decades has forced the field to revisit whole-phage therapy as a means for addressing especially resistant infections. Animal infection models confirm both the safety and efficacy of whole-phage therapy, and suggest that phage therapy could become a safe and effective supplement to antibiotics in the West17. However, the one-size-fits-all mindset that originally put phage therapy into remission during the time of d’Herelle must be replaced by the understanding that novel phage-based therapeutics will need to be developed frequently for an ever-changing microbial landscape. This changing mindset, however, is beginning to pervade many areas in healthcare with the rise of personalized medicine.
Recent advances in recombinant DNA technology have opened new doors for the creation of genetically-modified phages. For example, researchers at MIT recently engineered T7 coliphages to express an extracellular matrix-degrading enzyme in order to better destroy bacterial biofilms. Because biofilms are often highly resilient in the face of antibiotics, this work demonstrates a novel technique in combatting infections exhibiting biofilms18. Because phages are biological agents that contain nucleic acids (unlike antibiotics), they are highly malleable and can be engineered to fit the needs of specific applications19.
Finally, researchers have also isolated various phage proteins for use as independent therapeutics; two major classes of such proteins are endolysins and depolymerases. Endolysins facilitate the virulent phage lifecycle by breaking down peptidoglycan in the host’s cell wall to cause lysis, but recent research suggests that this protein can actually be used independently as a lysis-causing antibacterial peptide20. Because endolysin generally has less target specificity than the phages that encode them, this could be a method for addressing the limited host range exhibited by many phage-based therapeutics. The second phage protein currently of interest to many researchers is depolymerase, a class of enzymes that degrade bacterial capsules and lipopolysaccharides (LPS). Because LPS constitutes a major defense mechanism used by bacteria to resist antibiotics and other stressors, this enzyme is capable of enhancing the therapeutic effect of antibiotics, and has even been shown to revert antibiotic-resistant bacteria back to a state of drug sensitivity21, 22.
Conclusion
As the medical community’s antibiotic arsenal dwindles, it becomes more and more apparent that an alternative antibacterial therapy is necessary. Although initially discovered in 1917 and ultimately abandoned by the West in the 1930s, bacteriophages have the potential to fill this niche due to several key intrinsic advantages: their rapid discovery, low toxicity, high host specificity, and manageable resistance. A recent surge in literature surrounding novel clinical applications of phage-based therapeutics suggests that the field of phage therapy—100 years after its inception—is currently experiencing a renaissance in Western medicine and has great potential to become a leading 21st century antibacterial.
References
1. Young, R., personal communication, 22 June 2016.
2. Rohwer F, et al. (2009). Roles of viruses in the environment. Environ Microbiol 11(11):2771–2774.
3. Hyman P, Abedon ST. Bacteriophage host range and bacterial resistance. Adv Appl Microbiol.2010;70:217–248.
4. Skurnik M, Pajunen M, Kiljunen S. Biotechnological challenges of phage therapy. Biotechnol Lett.2007;29:995–1003.
5. Carlton, R. M. (1999). "Phage therapy: past history and future prospects." Arch Immunol Ther Exp (Warsz) 47(5): 267-274.
6. Abedon, S. T. and C. Thomas-Abedon (2010). "Phage therapy pharmacology." Curr Pharm Biotechnol 11(1): 28-47.
7. Kutter E, De Vos D, Gvasalia G, Alavidze Z, Gogokhia L, Kuhl S, et al. Phage therapy in clinical practice: treatment of human infections. Curr Pharm Biotechnol. 2010;11:69–86.
8. Górski A, Borysowski J, Miedzybrodzki R, Weber-Dabrowska B. Bacteriophages in medicine. In: McGrath S, van Sinderen D, editors. Bacteriophage: Genetics and Microbiology. Norfolk, UK: Caister Academic Press; 2007. pp. 125–158.
9. Capparelli R, Nocerino N, Iannaccone M, Ercolini D, Parlato M, Chiara M, et al. Bacteriophage therapy of Salmonella enterica: a fresh appraisal of bacteriophage therapy. J Infect Dis. 2010;201:52–61.
10. Skurnik M, Strauch E. Phage therapy: facts and fiction. Int J Med Microbiol. 2006;296:5–14.
11. Torres-Barceló, C. and M. E. Hochberg "Evolutionary Rationale for Phages as Complements of Antibiotics." Trends in Microbiology 24(4): 249-256.
12. Betts, A., et al. (2013). "Back to the future: evolving bacteriophages to increase their effectiveness against the pathogen Pseudomonas aeruginosa PAO1." Evol Appl 6(7): 1054-1063.
13. Fruciano DE, Bourne S. Phage as an antimicrobial agent: d’Herelle’s heretical theories and their role in the decline of phage prophylaxis in the West. The Canadian Journal of Infectious Diseases & Medical Microbiology. 2007;18(1):19-26.
14. Sulakvelidze, A., et al. (2001). "Bacteriophage Therapy." Antimicrob Agents Chemother 45(3): 649-659.
15. Eaton, M. D. and S. Bayne-Jones (1934). "Bacteriophage therapy: Review of the principles and results of the use of bacteriophage in the treatment of infections." Journal of the American Medical Association 103(23): 1769-1776.
16. Goodridge LD. Designing phage therapeutics. Curr Pharm Biotechnol. 2010;11:15–27.
17. Miedzybrodzki, R., Gorski, A., & Borysowski, J. (2014). Phage Therapy - Current Research and Applications. Haverhill, UK: Caister Academic Press.
18. Lu, T. K. and J. J. Collins (2007). "Dispersing biofilms with engineered enzymatic bacteriophage." Proceedings of the National Academy of Sciences 104(27): 11197-11202.
19. R. Citorik*, M. Mimee*, and T. K. Lu, “Bacteriophage-based synthetic biology for the study of infectious diseases”, Current Opinion in Microbiology, Volume 19, Pages 59-69, June 2014.
20. Nelson, D. C., et al. (2012). "Endolysins as antimicrobials." Adv Virus Res 83: 299-365.
21. Drulis-Kawa Z, Majkowska-Skrobek G, Maciejewska B, Delattre A-S, Lavigne R. Learning from Bacteriophages - Advantages and Limitations of Phage and Phage-Encoded Protein Applications. Current Protein & Peptide Science. 2012;13(8):699-722.
22. Sutherland IW, Hughes KA, Skillman LC, Tait K. The interaction of phage and biofilms. FEMS Microbiol. Lett. 2004;232:1–6.
23. Young, R. and J. J. Gill (2015). "Phage therapy redux—What is to be done?" Science 350(6265): 1163-1164.