After more than 150 years of studying Huntington disease, a potential cure may finally be in sight. On September 24th, uniQure, a biotechnology company located in Amsterdam, published its results for a novel treatment that slows down the progression of Huntington’s disease (HD).
What is Huntington’s Disease?
Huntington’s disease is a progressive neurodegenerative disorder that affects more than 75,000 people in the United States and Europe. The condition typically appears between the ages of 30 and 50, with symptoms worsening gradually over 10 to 25 years. Because of its hereditary nature, an estimated 200,000 Americans are at risk of developing the disease. As HD progresses, it causes steady decline in cognitive function, leading to gradual loss of both physical and mental abilities. Common symptoms include extreme mood swings, involuntary movements and memory loss.
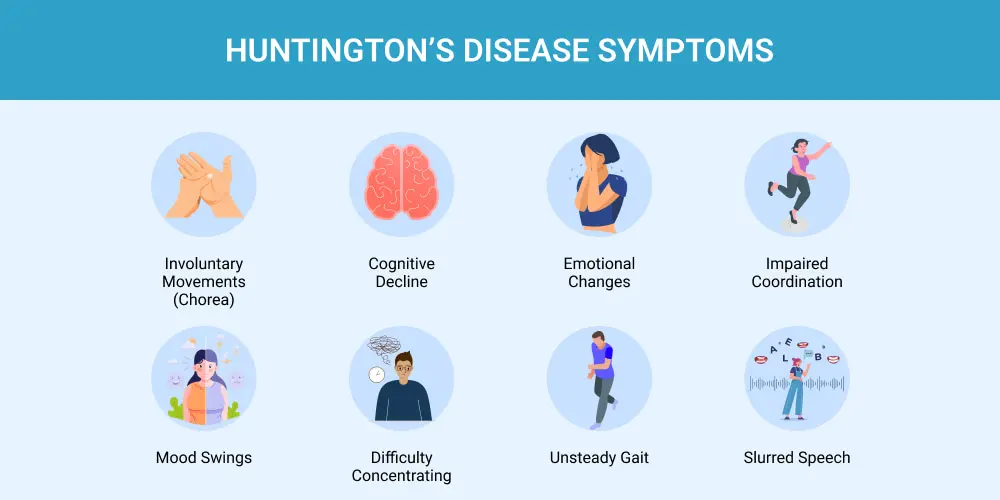
Above: Possible symptoms of Huntington’s disease. Image courtesy of Lone Star Neurology.
HD is a genetic disorder caused by a mutation in the huntingtin (HTT) gene. Specifically, Huntington’s disease is caused by excessive CAG nucleotide repeats in the gene’s DNA sequence. While people without HD typically have up to 26 repeats, those with 27-35 are at risk of developing the disease, and individuals with more than 36 almost always develop HD. The expanded CAG sequence disrupts the normal Huntingtin protein, ultimately damaging neurons and leading to cell death in the brain. The disease is inherited in an autosomal dominant manner, meaning that a child has a 50% chance of inheriting the condition if one parent carries a copy of the defective gene.
Currently, there is no cure for Huntington’s disease. Instead, doctors focus on managing symptoms through medications that control muscle movements or mood changes. These treatments aim to improve the patient’s quality of life, but they do not stop or slow the underlying neurodegeneration.
Recent Developments in Huntington’s Treatment
In a recent breakthrough study, researchers tested an experimental therapy called AMT-130, which targets the root cause of Huntington’s disease rather than just its symptoms. Early results suggest that this treatment drastically slows HD progression.
AMT-130 is considered a gene therapy. Specifically, it uses an artificial microRNA to bind to the defective huntingtin gene, silencing it and preventing faulty proteins from accumulating. To administer the treatment, neurosurgeons must conduct a one-time surgery to deliver AMT-130 directly into the inner-regions of the brain. However, because this surgery involves complex neural structures located below the cerebral cortex, the procedure is highly invasive and lengthy. Once AMT-130 has been delivered, it diffuses outward, until the microRNA effectively affects all neural cells.
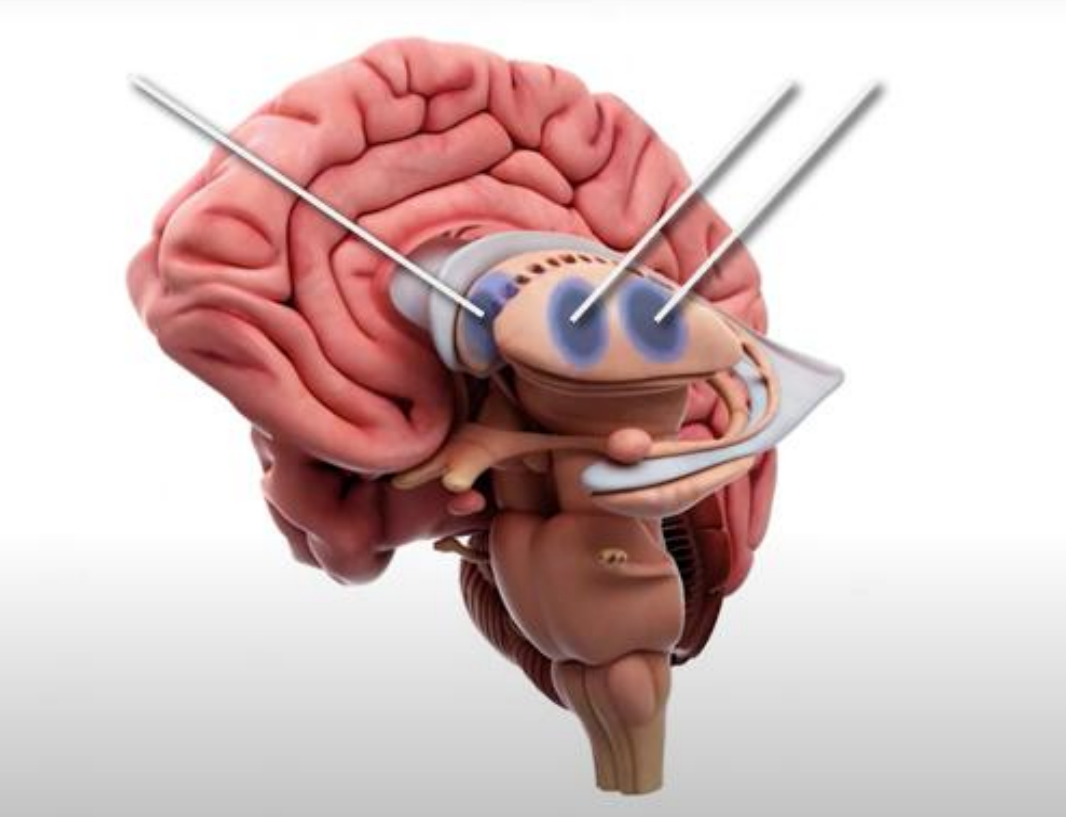
Above: Graphical representation of AMT-130 administration into the caudate nucleus and putamen of the striatum (inner-regions) of the brain for Huntington’s disease treatment. Image courtesy of uniQure.
On September 24th, uniQure published the results from their phase I/II clinical studies testing AMT-130 efficacy. These clinical studies measured brain function in 29 patients who exhibited early-stage Huntington-related decline. After 3 years of monitoring, researchers compared neurodegeneration of patients who were given a low-dose, a high-dose, or a placebo (serving as the control group).
For patients who received a high-dose of AMT-130, disease progression was slowed down by 75%. Scientists used cUHDRS, a scale used to diagnose HD progression, to measure patients’ motor and cognitive function over three years. Additionally, the scientists used a secondary test to confirm these results. They quantified the amount of neurofilament light (Nfl) protein in the cerebrospinal fluid, the liquid between the brain and the skull, as an additional measure for neurodegeneration. They found that patients with the treatment had lower levels of Nfl, suggesting that fewer neurons died.

Above: Motor and cognitive function tests conducted by uniQure to measure Huntington’s disease progression. Image courtesy of uniQure.
Following the success of these clinical trials, uniQure hopes AMT-130 can bypass phase III clinical studies, allowing the treatment to reach patients sooner. Traditionally, phase III clinical studies are used to ensure patient safety and compare the new treatment to a standard of care (the currently used treatment). However, since there are no existing treatments for Huntington’s disease, uniQure believes they can avoid some of this lengthy process. AMT-130 has already received the Breakthrough Therapy and Fast Track designation from the FDA, which would expedite the process.
What’s Next?
Although AMT-130 is a promising treatment for Huntington’s disease, there are some critical downsides. Due to the nature of gene therapies, AMT-130 treatment will likely be very costly, estimated at around $2 million per patient. This lofty price tag would greatly limit how many patients would be able to receive it. Additionally, some patients involved in the clinical trial experienced complications. Immediately after the surgery, three patients reported temporary but serious neurological side effects, such as brain swelling and headaches. Due to these effects, some are concerned about its ability to receive FDA approval.
At the same time, other pharmaceutical companies have been working on their own HD therapeutics. Most notably, Novartis Pharmaceuticals and SkyHawk Therapeutics are in clinical trials for oral drugs that could be taken by pill–a much easier method of drug delivery compared to invasive surgery. Overall, uniQure’s AMT-130 has shown promise in slowing the progression of Huntington’s disease, offering new hope for the future of HD treatment.
