
Above: Photo courtesy of StatNews.
When a new drug is declared “safe and effective,” most of us assume it will work for people like us. But what if it doesn’t? Clinical trials are the backbone of medical innovation, and yet the randomized controlled trial (RCT) model faces growing scrutiny. RCTs, put simply, evaluate the efficacy of new treatments by randomizing participants into control and treatment groups. The random aspect allows researchers to infer a causal relationship between treatment and outcome without bias or placebo effects. While RCTs are optimized to reduce bias using tightly controlled conditions, their inclusion/exclusion criteria can limit patient diversity in the trial population.
Consequently, RCTs create fundamental tension between minimizing bias and including real-world patients who fall outside the eligibility guidelines. For example, pregnant women are routinely excluded from clinical trials due to confounding variables or ethical challenges. Therefore, the results of the clinical trial cannot be generalized to include pregnant women, which may not be representative of the true patient population. As medicine shifts towards an age of personalization, scientists are exploring new tools to increase trial inclusivity.
One of the most promising tools to improve predictions is a digital twin: a virtual model that can simulate reactions to various conditions. Digital twins have been used to optimize products across industries such as manufacturing, energy, and healthcare. The concept of a digital twin was first realized in the 1960s by the National Aeronautics and Space Administration (NASA), not for human subjects, but for technology. NASA designed a “digital twin” for a spacecraft to address flight issues. NASA famously used a digital twin during the Apollo 13 mission to simulate the rocket's malfunction and determine a safe route back to Earth.
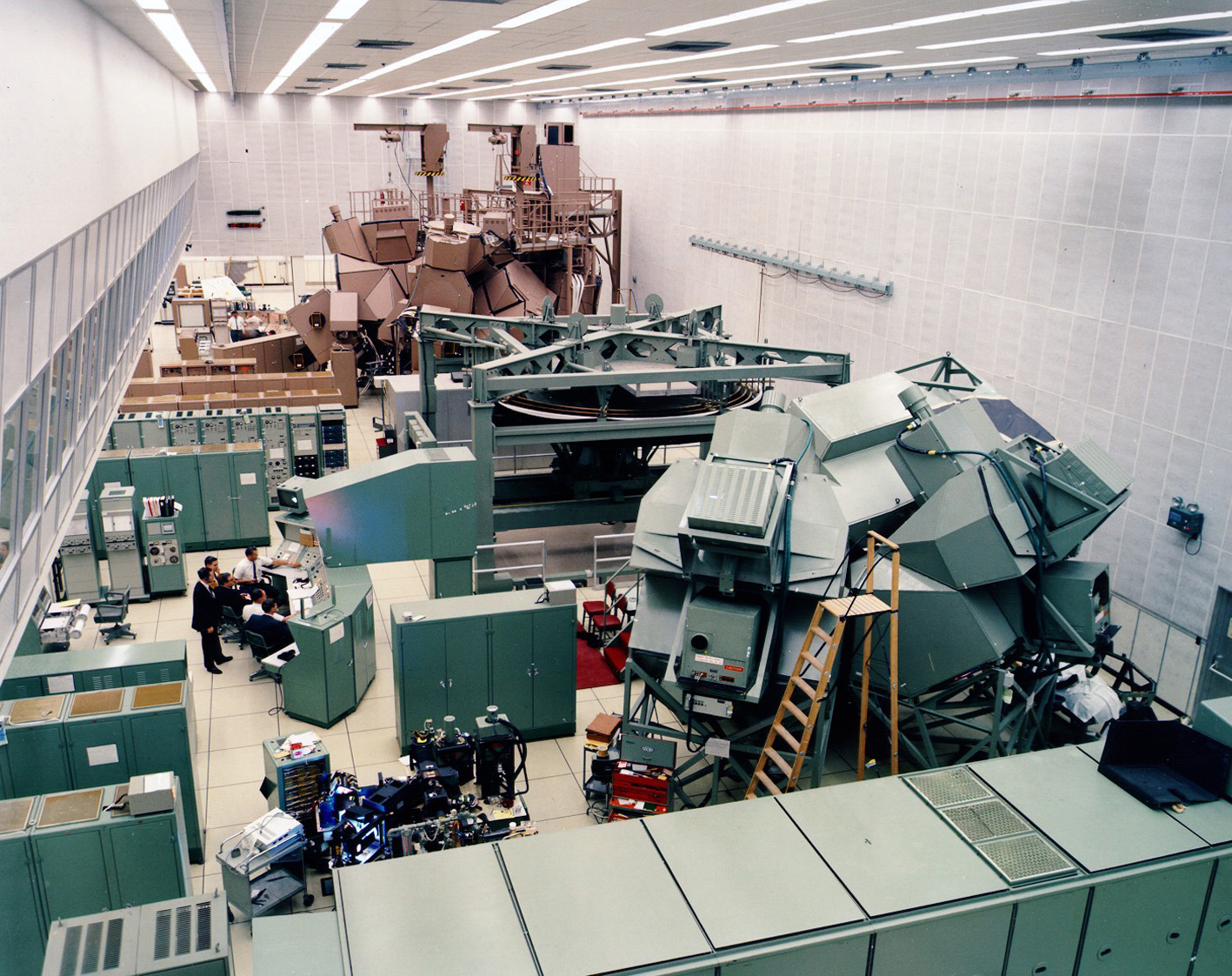
Above: The first digital twins used to simulate Apollo 13. Photo courtesy of Siemens.
Digital twins have since been adopted in healthcare. A review article defines a digital twin for healthcare as “a virtual representation of a person which allows dynamic simulation of potential treatment strategy…based on multi-scale modeling of multi-modal data such as clinical, genetic, molecular, environmental, and social factors” (Katsoulakis, E. et. al, 2024). In clinical trials, digital twins generated from rich patient datasets offer a way to emulate more realistic patient populations without adding recruitment burden or harboring the same ethical concerns as human trial participants.
Above: Representation of a digital twin, courtesy of Unlearn AI.
Creating a digital twin begins with the collection of extensive biological and clinical data, including genetic profiles, biomarkers, and lifestyle information. These data are then integrated with historical datasets to build generalized, representative virtual patient populations. Ultimately, these virtual patients can be divided into cohorts and undergo predictive quantitative systems pharmacology (QSP) modeling to simulate treatment with the drug candidate. This allows researchers to simulate how a drug candidate might behave across a broader, more diverse population than that captured by a traditional clinical trial.
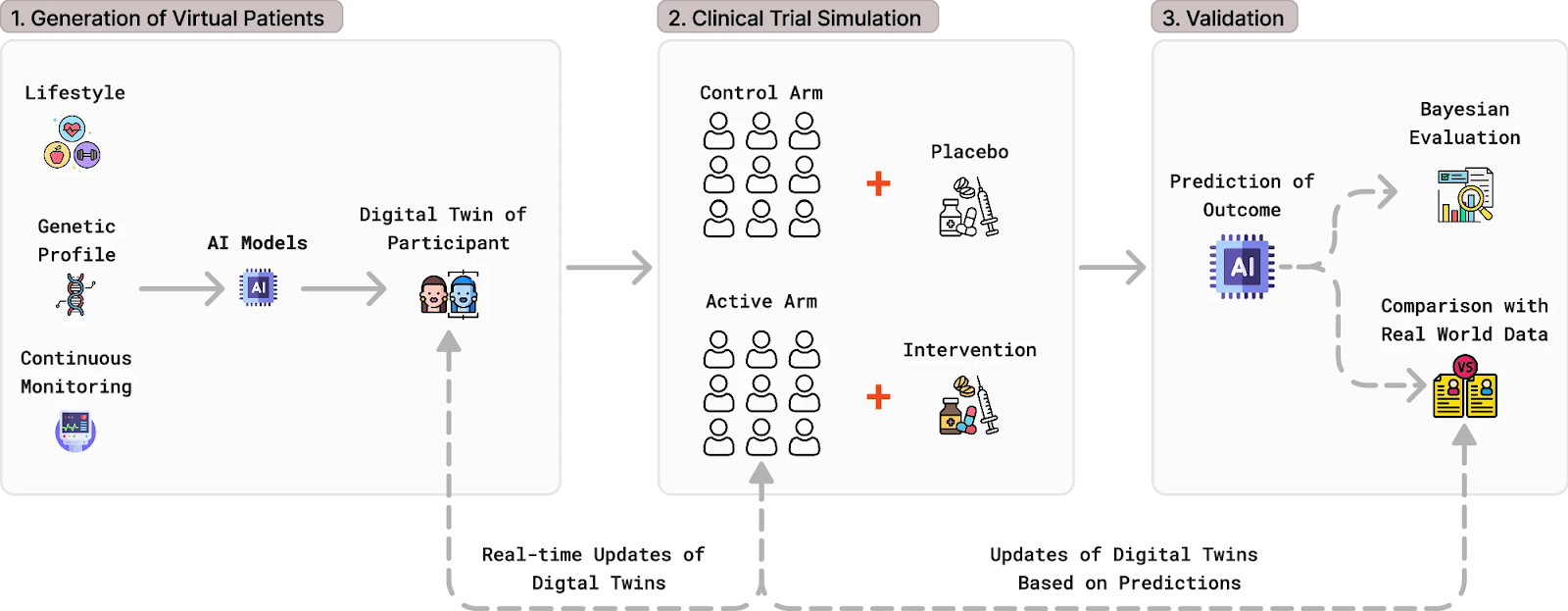
Above: Workflow of digital twins in clinical trials. Courtesy of NPJ Systems Biology and Applications.
Pharmaceutical companies have recognized the promise of digital twins to streamline drug testing and approval. Digital twins can reduce the number of control patients needed in a clinical trial, saving money on enrollment costs and significantly shortening trial timelines. The impact of digital twinning has been most noted in areas of research that struggle to recruit patients for trials. For example, it is difficult to recruit patients for Alzheimer’s research due to the likelihood of comorbidities in the elderly population that may bias the results of the trial. At the Alzheimer’s Association International Conference in 2024, the digital twin company Unlearn presented that “Digital twins could reduce control arm sizes by 33% when estimating the treatment effect” on Alzheimer’s markers. This reduction in sample size would, in turn, save millions of dollars on late-phase trials.
It is important to note that digital twins are not replacing human trial participants. Rather, these simulated patients fill critical gaps in the current RCT model by reducing timelines and costs and allowing for more real-world and generalizable clinical data. While challenges with data quality and regulatory acceptance remain in this field, the momentum behind digital twins continues to build as more pharmaceutical companies continue to optimize and validate these models.
